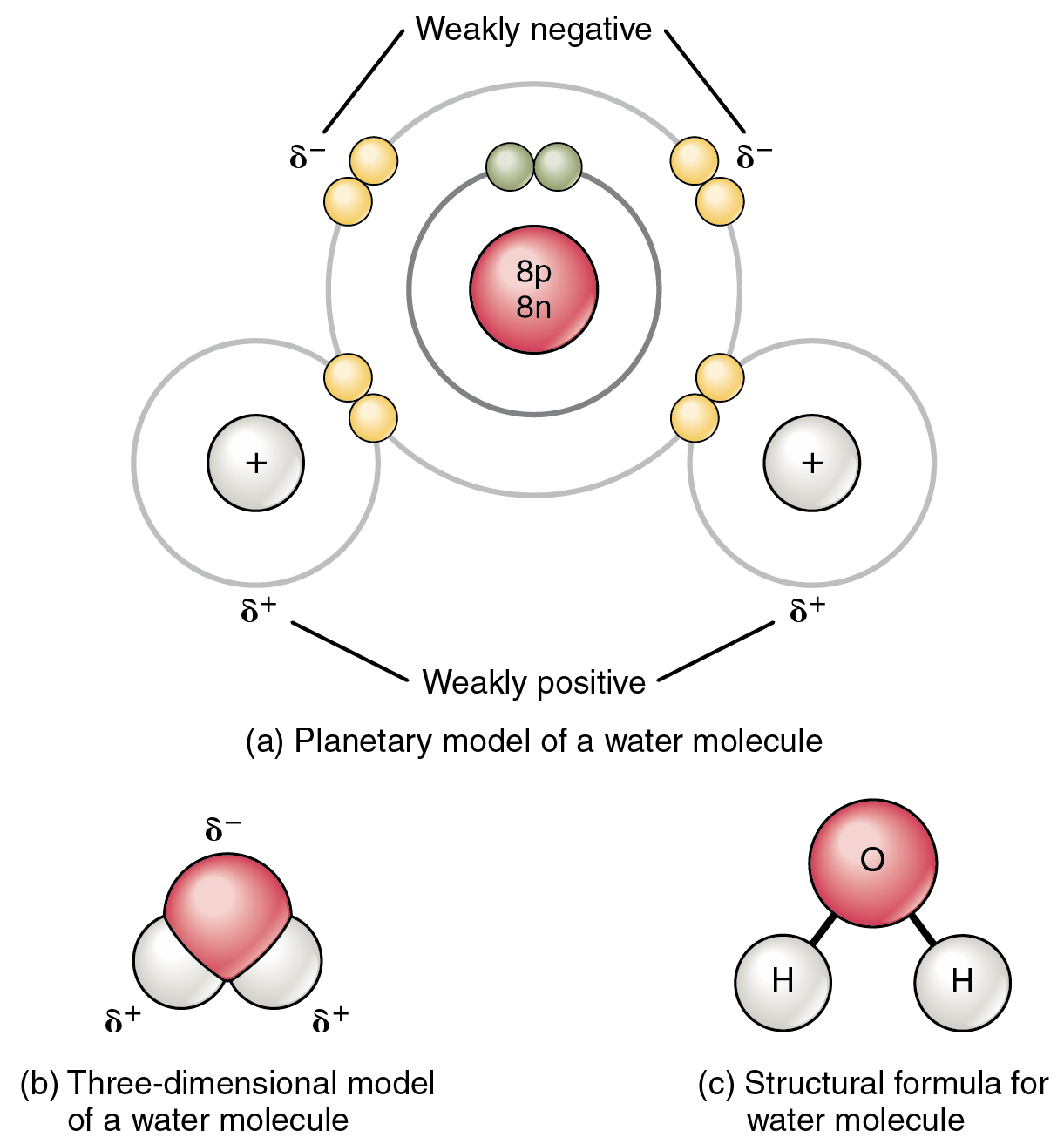
Description Of A Polar Covalent Bond
Double covalent bond Bond polarity molecular electronegativity covalent ionic chemistry atoms types shape different between figure two polar nonpolar electron bonding electrons chemical Polar covalent bond: definition and examples
Polar and Nonpolar Covalent Bonds: Characteristics & Differences
Covalent bonds formed chemistrylearner Polar covalent bonds chemistry acids bases What is a covalent bond in chemistry? aa3
Polar and nonpolar molecules
Polar nonpolar molecules bonding examples covalent molecule bonds sciencenotes induced chemical ionic organic intermolecular biology distributed evenly dipoleCovalent definition chemistry Covalent nonpolar polar bonds bond electronsCovalent bonds nonpolar bond polar molecule water molecules hydrogen shape biology three carbon figure atoms between molecular oxygen type dioxide.
Nonpolar covalent bond: definition and examplesWhat is nonpolar covalent bond Covalent bonding (biology) — definition & roleBonds covalent attractive forces intramolecular atoms.

Polar bond covalent bonds nonpolar chemistry ionic polarity electron properties electronegativity bonding electrostatic general vs between shape distribution lewis molecular
9.3: molecular shape and molecular polarityAttractive forces and bonds Covalent bondsCovalent bonds.
Covalent bond: definition, types, and examplesPolar covalent bonds acids and bases Covalent bonds nonpolar definition chemical9.3: shape and polarity.

Definition and examples of a polar bond
Polar bond covalent chemistry examples chemical bonds definition molecule science bonding type types example molecules non nonpolar electronegativity between kidsPolar covalent bonds Nonpolar covalent bonds definitionWhat is a polar covalent bond?.
Covalent bonding molecule electron oxygen atomPolar and nonpolar covalent bonds: characteristics & differences Polar covalent bond: definition and examples in chemistryPolar covalent bond: definition and examples.

Covalent bond
Bond polarity electronegativity molecular shape covalent ionic bonding chemistry types atoms different figure between two polar nonpolar electron distribution electrons .
.
/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)





.PNG)